
For example, a white enhancer in an Mcp transgene can activate a white reporter in a second Mcp transgene inserted several thousand kb away. The first findings supporting an architectural function came from the discovery that su(Hw) and a BX-C boundary, Mcp ,mediate long-distance regulatory interactions.
#LOOPED DOMAINS WITH PROTEIN SCAFFOLD SERIES#
īoundaries are architectural elements that define topologically independent domainsĪs initially envisioned, the primary function of boundaries is architectural they subdivide the chromosome into a series of topologically independent looped domains. For example, human chromosomes have over 15,000 cell type-independent sites for the CTCF boundary protein, as well as many cell type-specific sites. While fewer than 100 or so have actually been characterized, ChIP experiments suggest that there are thousands in chromosomes of multicellular eukaryotes.

Many other boundaries have subsequently been discovered in organisms ranging from yeast ( S. They flank the 87A7 heat shock locus and are located close to the borders of the “puff” of decondensed chromatin that forms when the hsp70 genes are induced. Two other boundaries, scs and scs', were identified molecularly. This protein binds to multiple sites in su(Hw) and is responsible for its blocking activity. These mutagenic effects can be suppressed by mutations in the gene encoding the Su(Hw) protein. A large percentage of spontaneous mutations in flies are gypsy insertions that block normal enhancer-promoter interactions. The third boundary, su(Hw), is in the gypsy retrotransposon. In the former case, the iab-6 initiator activates iab-7 inappropriately in PS11, while in the latter case, the iab-7 Polycomb Response Element (PRE) silences iab-6. In Fab-7 mutants, the two domains fuse, and as a consequence, cells in PS11 (A6) assume either a PS12 (A7) or a PS10 (A5) identity. One function of this boundary is to block adventitious interactions between initiators, enhancers and Polycomb-dependent silencers in the two regulatory domains. The second boundary, Fab-7, is located between two regulatory domains in the Bithorax complex (BX-C), iab-6 and iab-7, which direct the expression of the Abd-B gene in parasegments 11(PS11/abdominal segment A6) and 12 (PS12/A7), respectively. It alters the banding pattern in polytene chromosomes, fusing the band that contains Notch with the adjacent band, and causing a chromosomal position effect that reduces Notch expression. One, facet strawberry is an ∼900 bp deletion upstream of the promoter of the Notch gene. The first such elements (called boundary elements or insulators) were discovered in Drosophila several decades ago. If chromosomes are subdivided into a series of precisely defined loops, a plausible inference is that there are special cis -acting elements that delimit the ends or boundaries of each loop. Further support came from EM micrographs of metaphase chromosomes by Laemmli and colleagues, which showed thousands of large loops anchored at their base by a scaffold-like structure. This model was supported by biochemical studies of Benyajati and Worcel which suggested that Drosophila chromosomes are organized into loops of about 80 kb.
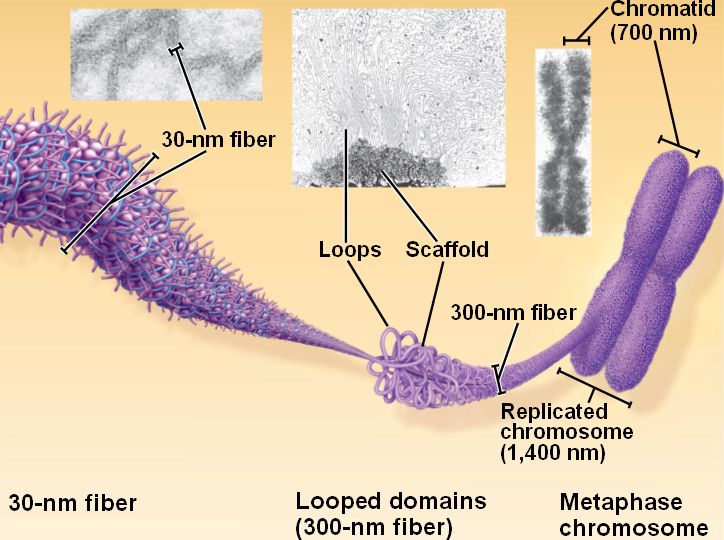
These examples gave rise to the idea that chromosomes in somatic cells are subdivided into a series of looped domains. Like polytenes, the looping pattern is dictated by the underlying DNA sequence. The loops are arranged in pairs, one from each chromatid. Emanating from the condensed chromatin that forms the main axis of the chromosome are loops that contain actively transcribed genes. At this stage, the two sister chromatids are paired in register.
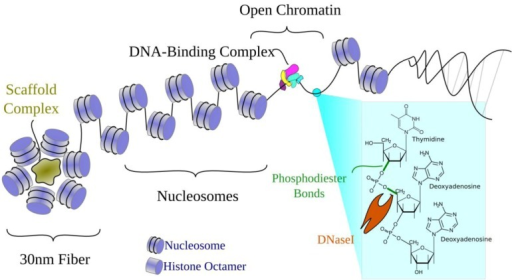
Moreover, analysis of deletion and inversion mutants shows that the banding pattern is dictated bythe underlying DNA sequence.Īnother classic example found in both invertebrates and vertebrates are the lampbrush chromosomes of oocytes arrested at the diplotene stage of meiosis I. This pattern (bands and interbands) is highly reproducible from one cell to the next and between individuals. The resulting chromosomes have banding patterns that reflect the relative degree of compaction in each chromosomal segment. The homologs are also paired, again in register. The euchromatic regions of these polytene chromosomes are replicated tens to hundreds of times, and the copies are aligned in precise register. One classic example are the polytene chromosomes in Diptera salivary glands, fat bodies and nurse cells. The first evidence that the chromatin fiber in chromosomes of multicellular eukaryotes has a regular, stereotypic 3D organization came from cytological studies dating back more than 100 years. The 3D organization of the genome in multicellular eukaryotes is thought to play a crucial role in regulation, recombination and repair.


 0 kommentar(er)
0 kommentar(er)
